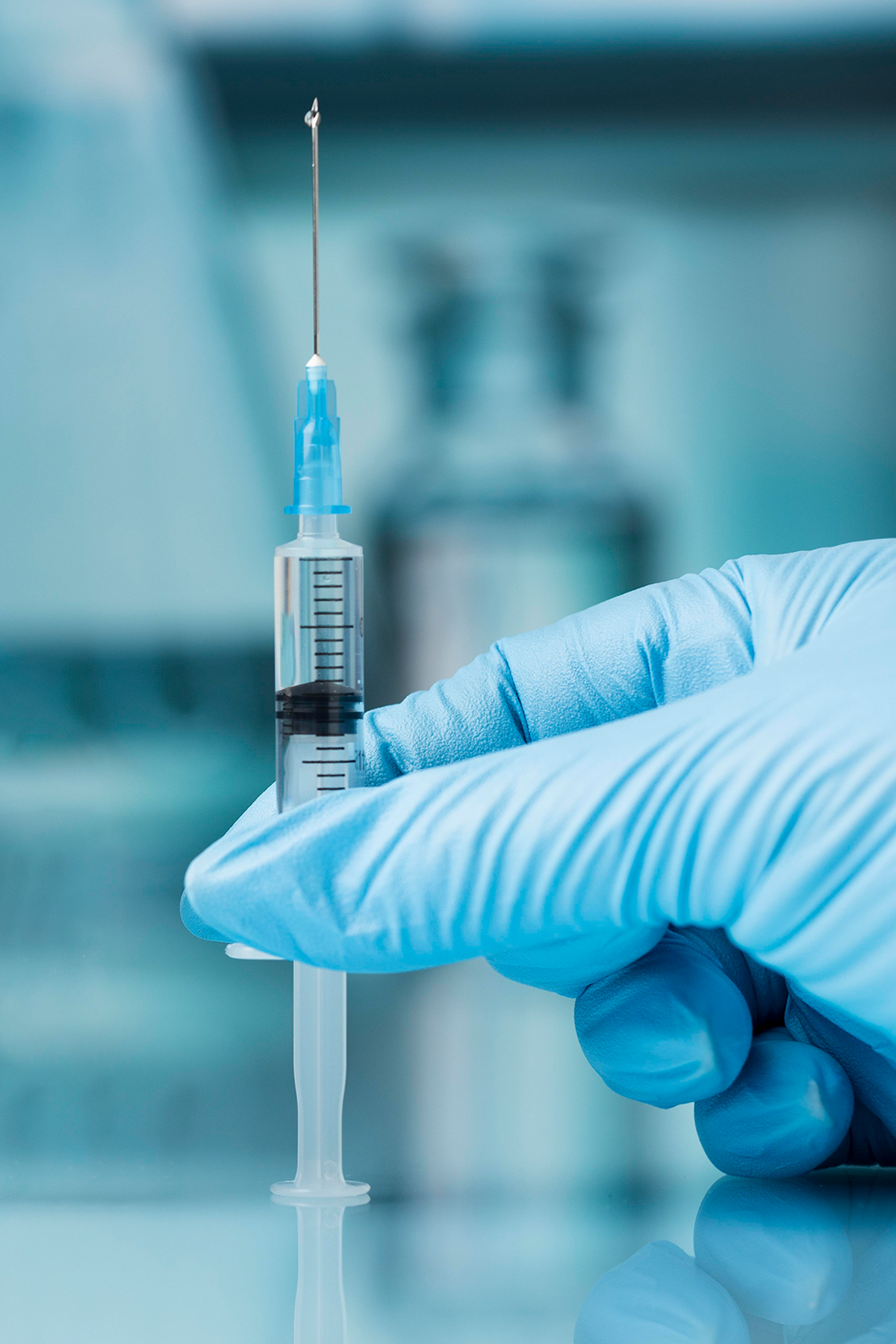
SecureGard®
Advantages of choosing SecureGard retractable syringe
- Low dead space (LDS) – reduce drug wastage
- No change in technique, requires minimal training
- Manual one-handed retraction
- Low cost solution
- Latex free
- Breakaway plunger
- Available in 1ml, 3ml and 5ml capacity
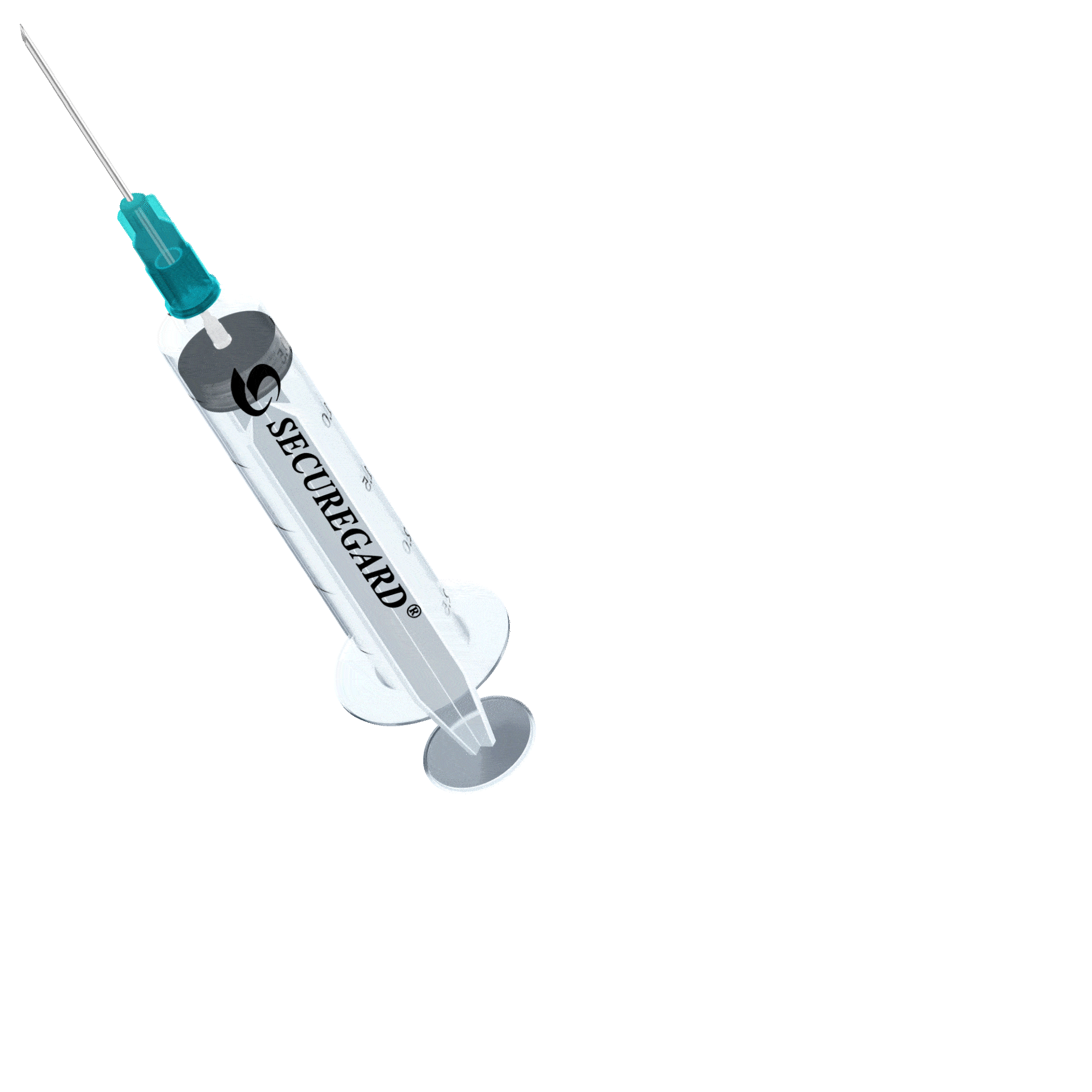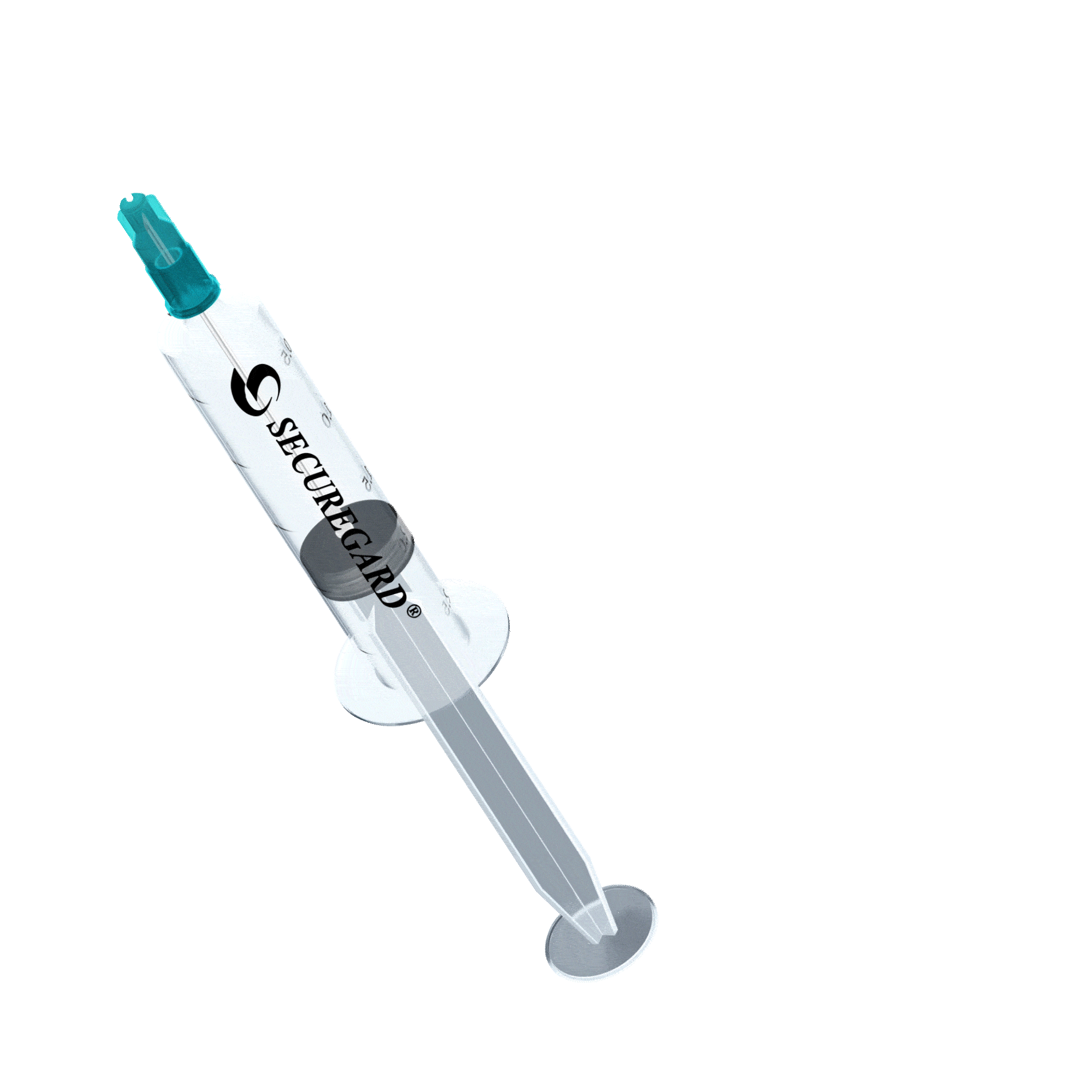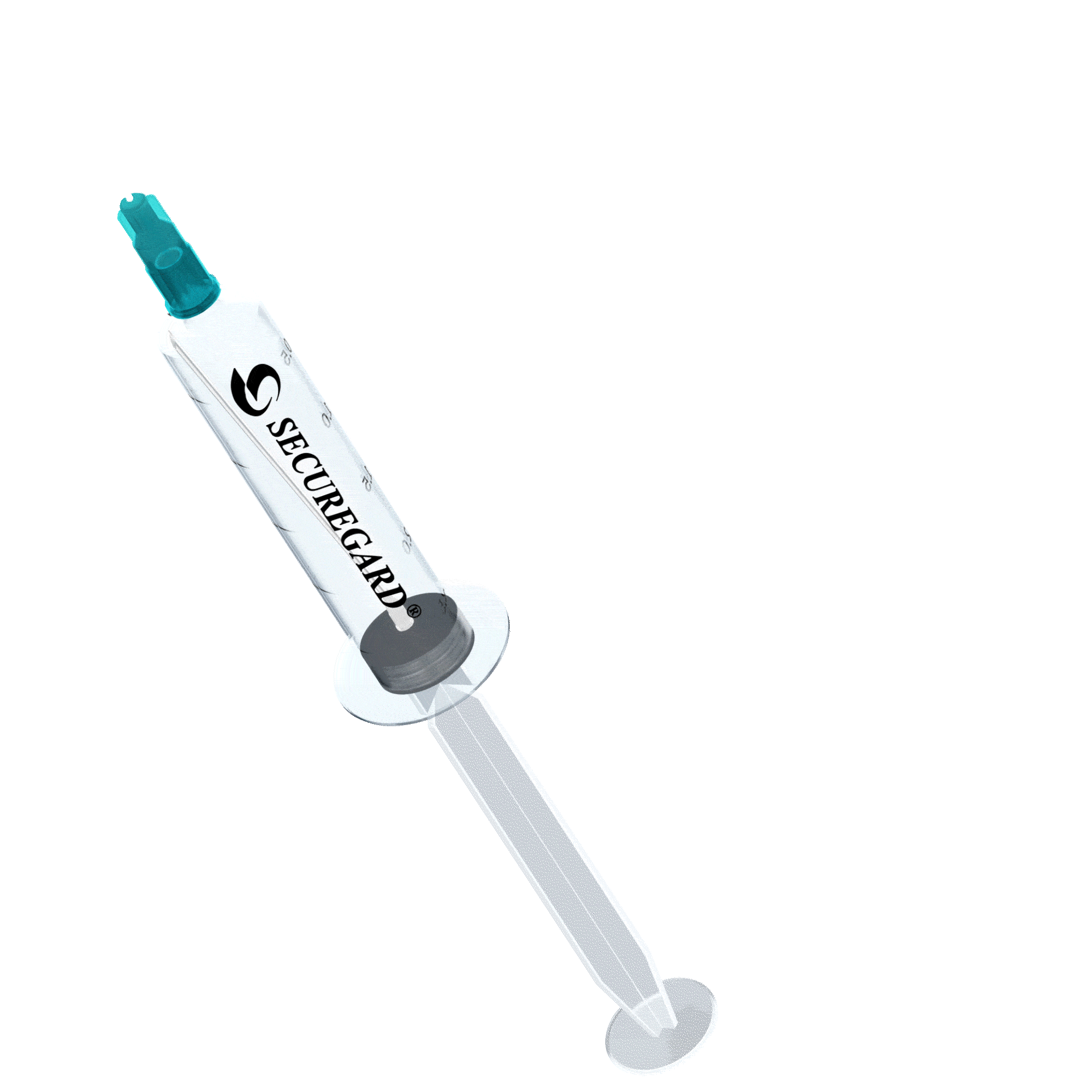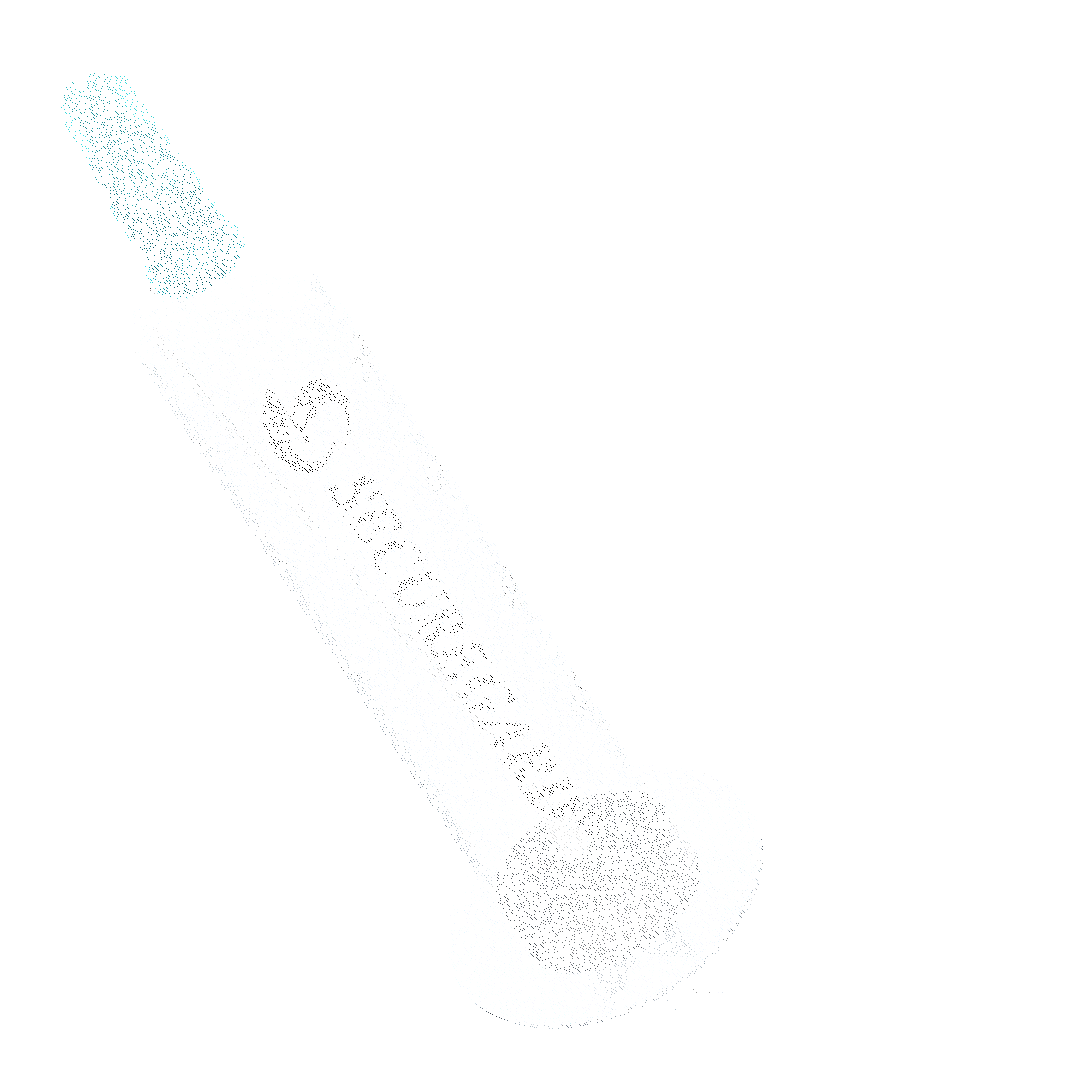
6 Steps for safe injections with SecureGard
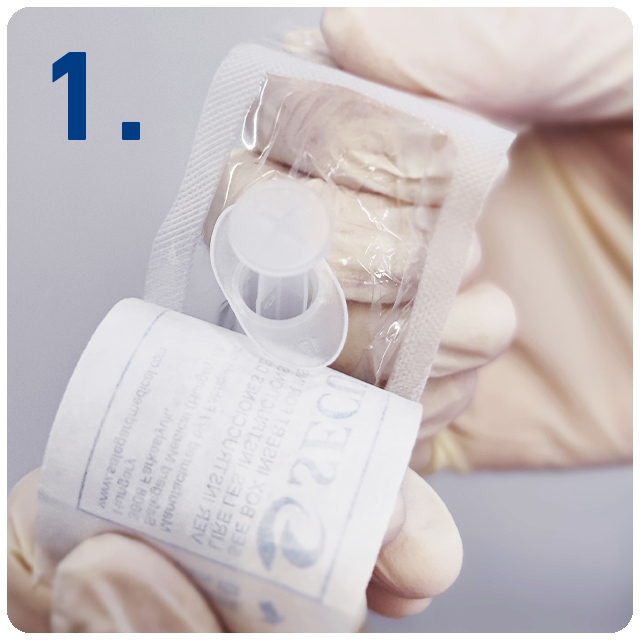
Open at peel.
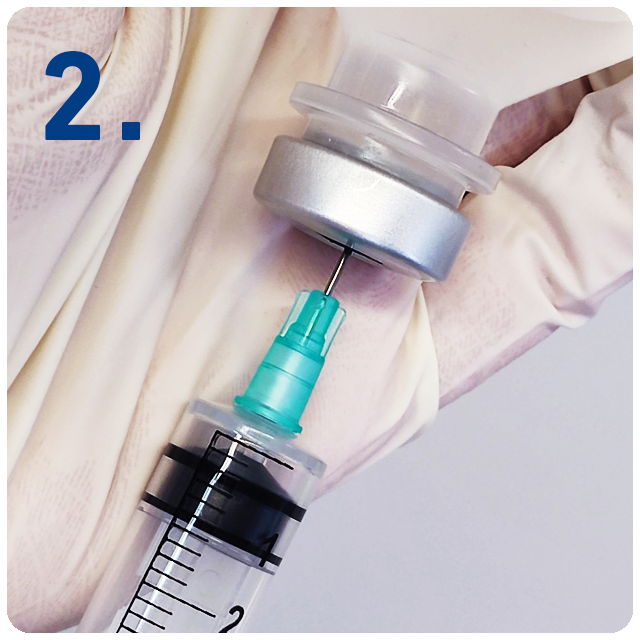
Remove protective needle cover and use standard technique to draw up medication.
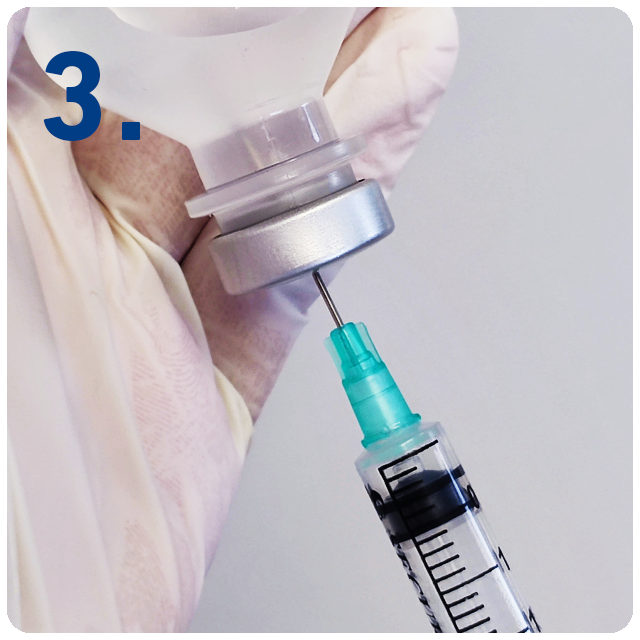
Expel air into the vial if necessary, ensuring the plunger is not depressed beyond the first graduation mark on the syringe.
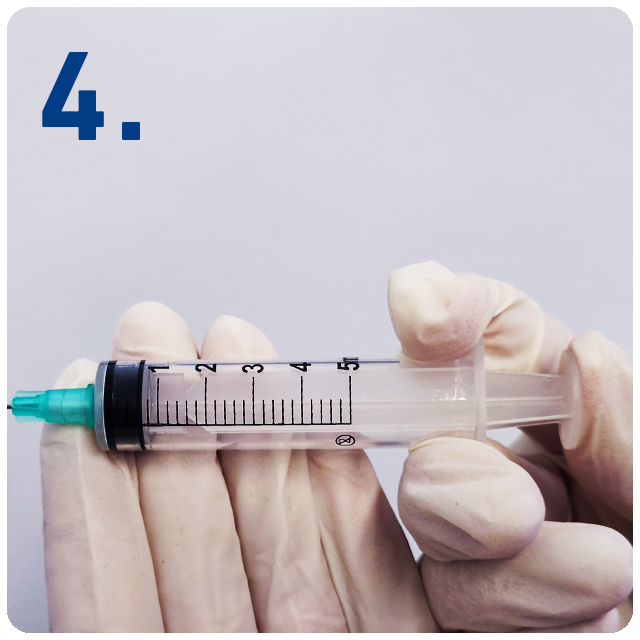
Inject the medication by fully depressing the plunger.
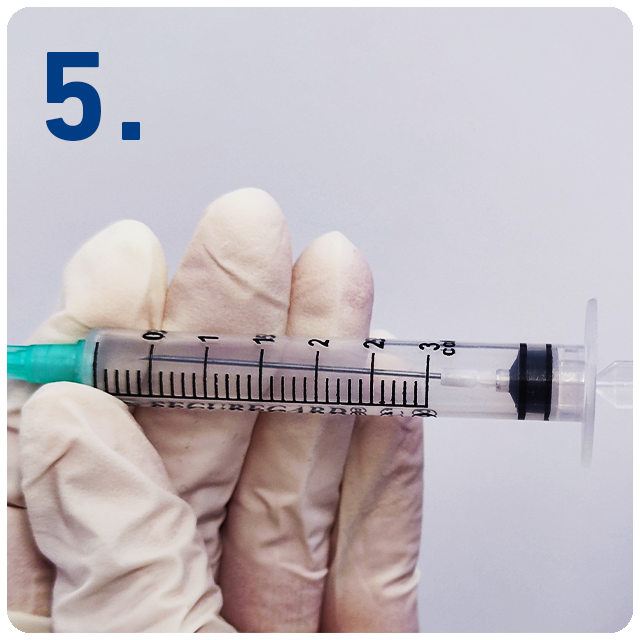
Draw back plunger to retract the needle safely into the barrel of the syringe.
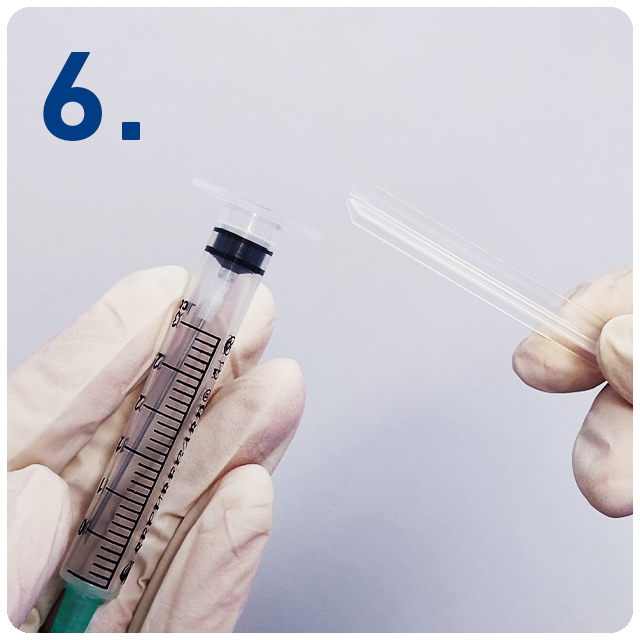
Break off the plunger at the break point and dispose.
Technical Information
The SecureGard® Retractable Safety Syringe is a single-use, sterile, disposable hypodermic syringe with a needle attached which is intended for dispensing/administering fluids in medical practice.
The key safety feature, where the clinician retracts the hypodermic needle by manually locking the plunger onto the needle hub, withdrawing the plunger, pulling the needle into the barrel and breaking the plunger, virtually eliminates the accidental clinical reuse of the syringe and accidental needle stick injuries.
The syringe is presently manufactured in 1.0, 3.0, and 5ml capacities. The table below illustrates the availability of syringe/needle combinations ‘off the shelf’.
| 5311W | Yellow | Securegard 1ml with 30Gx1/2″ needle attached insulin U100 | ||||
| 5311I | Red | Securegard 1ml with 29Gx1/2″ needle attached insulin U100 | ||||
| 5311X | Green-Blue | Securegard 1ml with 28Gx1/2″ needle attached insulin U100 | ||||
| 5311J | Medium Gray | Securegard 1ml with 27Gx1/2″ needle attached insulin U100 | ||||
| 5318A | Red | Securegard 1ml with 29Gx1/2″ needle attached | ||||
| 5318X | Green-Blue | Securegard 1ml with 28Gx1/2″ needle attached | ||||
| 5318B | Medium Gray | Securegard 1ml with 27Gx1/2″ needle attached | ||||
| 5318D | Orange | Securegard 1ml with 25Gx5/8″ needle attached | ||||
| 5318R | Orange | Securegard 1ml with 25Gx1″ needle attached | ||||
| 5348D | Orange | Securegard 3ml with 25Gx5/8″ needle attached | ||||
| 5348R | Orange | Securegard 3ml with 25Gx1″ needle attached | ||||
| 5348E | Deep Blue | Securegard 3ml with 23Gx1″ needle attached | ||||
| 5348F | Deep Blue | Securegard 3ml with 23Gx1 1/4″ needle attached | ||||
| 5348Q | Black | Securegard 3ml with 22Gx1″ needle attached | ||||
| 5348G | Black | Securegard 3ml with 22Gx1 1/4″ needle attached | ||||
| 5348U | Black | Securegard 3ml with 22Gx1 1/2″ needle attached | ||||
| 5348P | Deep Green | Securegard 3ml with 21Gx1″ needle attached | ||||
| 5348M | Deep Green | Securegard 3ml with 21Gx1 1/4″ needle attached | ||||
| 5348H | Deep Green | Securegard 3ml with 21Gx1 1/2″ needle attached | ||||
| 5158E | Blue | Securegard 5ml with 23Gx1″ needle attached | ||||
| 5158F | Blue | Securegard 5ml with 23Gx1 1/4″ needle attached | ||||
| 5158Q | Black | Securegard 5ml with 22Gx1″ needle attached | ||||
| 5158G | Black | Securegard 5ml with 22Gx1 1/4″ needle attached | ||||
| 5158P | Deep Green | Securegard 5ml with 21Gx1″ needle attached | ||||
| 5158M | Deep Green | Securegard 5ml with 21Gx1 1/4″ needle attached | ||||
Quantities per Box – 100 pieces
Quantities per Case – 1000 pieces
| Device | ||
| Barrel | Medical Grade Polypropylene | |
| Plunger | Medical Grade Polypropylene | |
| Grommet | Medical Grade TPE | |
| Inner Hub | Medical Grade POM | |
| Outer Hub | Medical Grade Polypropylene * | |
| Needle | 304 grade | |
| Sheath | Medical Grade Polypropylene | |
All products are Latex free.
(*coloured with colour masterbatch)
| Blister | Laminated polypropylene/polyethylene film |
| Paper | Royal Medical G 60gsm |
| Dispensing Carton | Corrugated cardboard |
| Shipping Carton | Corrugated cardboard |
Securegard® products are bio-compatible and sterilised by using a validated ETO gas sterilisation process. Syringes are meet the ISO 7886-1 Standard requirements, except the Luer-connection, and also meet the MDD 93/42 EEC requirements.
Frequently asked questions
Draw up the drug to be given in the usual manner.
- Using a standard one-handed technique, give the injection in line with your current clinical practise.
- Remember to fully depress the plunger when you are giving the injection thus capturing the safety needle (“clicks” when engaged).
- Retract the needle from the patient back into the barrel.
- Withdraw the plunger up to the “stop” at the top of the barrel.
- Break the plunger off, capturing the needle and ensuring that the syringe cannot be reused.
- Dispose of the Safety Syringe into a sharps container.
It is true to say that it was common clinical practise to use two needles when giving an injection. We believe it is no longer necessary to carry out this procedure. This view is supported more and more by clinicians in the field during our trials of SecureGard®.
- The high quality of the steel used in the production of the needles, the vastly improved needle sharpness means that the needles suffer almost no damage during “draw-up”.
- The improved siliconisation of the needle reduces the friction during injection and minimises patient discomfort.
- Also, there is the risk of an accidental needlestick when changing to the second needle.
- You must however ensure that the SecureGard® needle is attached before you give the injection.
- The retraction mechanism will only function with SecureGard® needles attached.
As with all clinical practises some care has to be taken.
The SecureGard® Safety needle is welded in place thus ensuring that it will not come loose prior to giving the injection.
- The special needle used to deliver the injection is held in the needle hub.
- The tip of the plunger is specially modified to engage the needle.
- The final movement of the plunger, at the end of drug delivery, enables the plunger to “grab” the needle.
- Retraction of the plunger withdraws the needle safely back into the barrel.
- After retraction the needle falls to the side within the barrel, preventing re-deployment.
- Once the needle is fully inside the barrel, the plunger is broken off, preventing re-use of the syringe.
One.
With the needle “captured” simply retract the plunger to withdraw the needle safely into the barrel.
Yes if you so wish to.
By moving the plunger back and forward gently this will allow you to check the movement without accidentally activating the mechanism.
Remember not to depress the plunger fully.
Fill the syringe and deliver the drug in the usual manner. Remember to fully depress the plunger to the end of travel to capture the needle, and then withdraw the needle into the barrel.
Click here to see diagrams to see how to use SecureGard®
No.
After the retraction mechanism has been triggered, the needle is held securely inside the barrel of the syringe.
Yes, absolutely!
Our unique design ensures that 1ml safety syringe can be built to the same exacting standards as other sizes in the range.
Safety and reliability of the product has been key to the success of the product.
Yes!
All SafeGard Medical products are latex free, thus ensuring total protection for the clinician.
Yes.
Just remember to fully depress the plunger firmly to activate the retraction mechanism and thereafter dispose of the used syringe according to hospital protocol.
As you know the dead space is the volume of drug, which remains in the syringe, following the completion of the clinical procedure.
- The Dead Space is compensated for on the scale of the barrel of the syringe.
- The dead space for all the SecureGard® Syringe meets the required ISO standards.
- Full details for each syringe can be found in the technical section.
According to US Federal Specification GG-N-196, the bevel styles used are defined
as style – “A”
Paraldehyde reacts with ALL plastic syringes. Not all manufacturers feel it is necessary to advise customers of this problem. We feel SecureGard® users should be given all the information about contraindications, even if it is highly unlikely they would ever use paraldehyde.
The SecureGard® Syringe has taken two years of extensive trailing and clinical testing in a number of countries worldwide to ensure that the product is of the highest possible quality standard. Thus ensuring total protection to the clinician.
The company Clinical Support Manager will discuss your exact training requirements and a customised programme will then be developed to meet the needs of your staff.
Get in touch with us today
Location
Farkaslyuk,
Jószerencsét tér 15,
3608 Hungary
Hungary
